
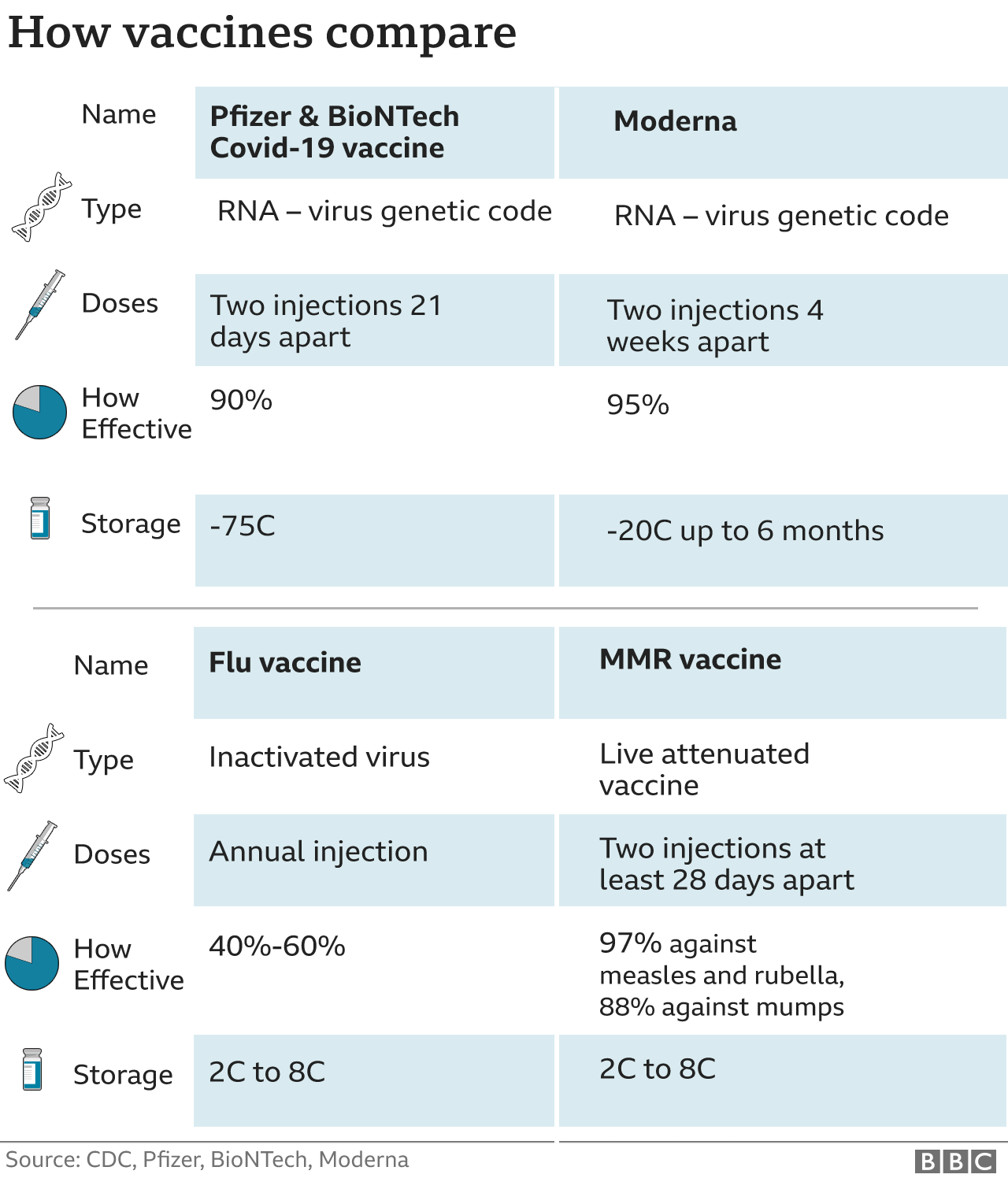
Tunis and his colleagues from the Public Health Agency of Canada presented their data about the delayed dosing schedule to the CDC’s vaccine advisers in early February, when the agency was considering extending the interval between doses.ĭr. “Eight weeks can create the opportunity to develop stronger and more broad immunity, which could be important in future waves of the pandemic,” Matthew Tunis, executive secretary for Canada’s National Advisory Committee on Immunization, told CNN. New vaccine demonstrates strong protection against severe Covid-19 in clinical trials (Photo by JOEL SAGET / AFP) (Photo by JOEL SAGET/AFP via Getty Images) Joel Saget/AFP/Getty Images

Several studies found that the delay lowered the already rare risk of myocarditis or pericarditis after vaccination, and there was an added benefit.Ī lab technician dedicated to the vaccines formulation, wearing Personal Protective Equipment (PPE), prepares stainless steel tanks for manufacturing vaccines preparations before the syringe filling phase, at a French pharmaceutical company Sanofi's world distribution centre in Val-de-Reuil on July 10, 2020. “The benefits for both mRNA vaccines far outweigh the risk of myocarditis compared to no vaccine,” Oliver said.Ĭanada opted to delay a second dose up to sixteen weeks in order to vaccinate more people when shots were in short supply, then later adjusted to an eight-week interval. Still, the benefits of receiving the Pfizer or Moderna vaccine are clear, regardless of the time in between shots, she said. Sara Oliver, an epidemic intelligence service officer with the Division of Viral Diseases, told the committee that rates of myocarditis were lower with extended intervals between first and second doses.
Pfizer second dose timing cdc series#
The CDC says the three- or four-week interval is still recommended for people who are moderately or severely immunocompromised, adults 65 and older “and others who need rapid protection due to increased concern about community transmission or risk of severe disease.” There’s no data around children younger than 11, so this group is still recommended to get the second Pfizer vaccine three weeks after the first dose.īooster doses continue to be recommended for most people five months after the two-dose primary series of an mRNA vaccine or two months after a Johnson & Johnson single-dose primary vaccination.Īt a meeting of the CDC’s independent Advisory Committee on Immunization Practices this month, agency officials suggested that guidance could be updated to recommend lengthening the interval between first and second doses of the mRNA vaccines. “An 8-week interval may be optimal for some people ages 12 years and older, especially for males ages 12–39 years,” the new guidance says. “While absolute risk remains small, the relative risk for myocarditis is higher for males ages 12-39 years, and this risk might be reduced by extending the interval between the first and second dose,” the CDC said, noting that some studies in people older than 12 have shown “the small risk of myocarditis associated with mRNA COVID-19 vaccines might be reduced and peak antibody responses and vaccine effectiveness may be increased with an interval longer than 4 weeks.” Because of omicron's increased transmissibility and ability to get around standard doses of vaccines, health officials have been strengthening their call for booster doses as maximum protection against COVID-19.People with Covid-19 may face long-term cardiovascular complications, study says The FDA's expansion of who can get a booster comes as the omicron and delta variants spike record numbers of COVID-19 cases in the US. No new safety concerns were reported to date in those individuals, the FDA said. In Monday's news release, the FDA said it reviewed real-world data from Israel in more than 6,300 children ages 12 to 15 who received a booster at least five months after their second dose of Pfizer.
"Following the FDA's authorizations, today's recommendations ensure people are able to get a boost of protection in the face of Omicron and increasing cases across the country, and ensure that the most vulnerable children can get an additional dose to optimize protection against COVID-19," CDC Director Dr. A committee that guides the CDC is scheduled to meet Wednesday to discuss whether to recommend third doses for all kids ages 12 and up. The FDA on Monday also authorized booster shots of Pfizer for all kids ages 12 to 15, but the CDC hasn't yet recommended them for that age group. The newest CDC guidance comes a day after the US Food and Drug Administration made the new recommendations for Pfizer's COVID-19 vaccine and booster.
The CDC also recommended a third dose of Pfizer's COVID-19 vaccine, the only one authorized for kids and teens, for children as young as age 5 who are " moderately or severely immunocompromised," 28 days after their second shot.


 0 kommentar(er)
0 kommentar(er)
